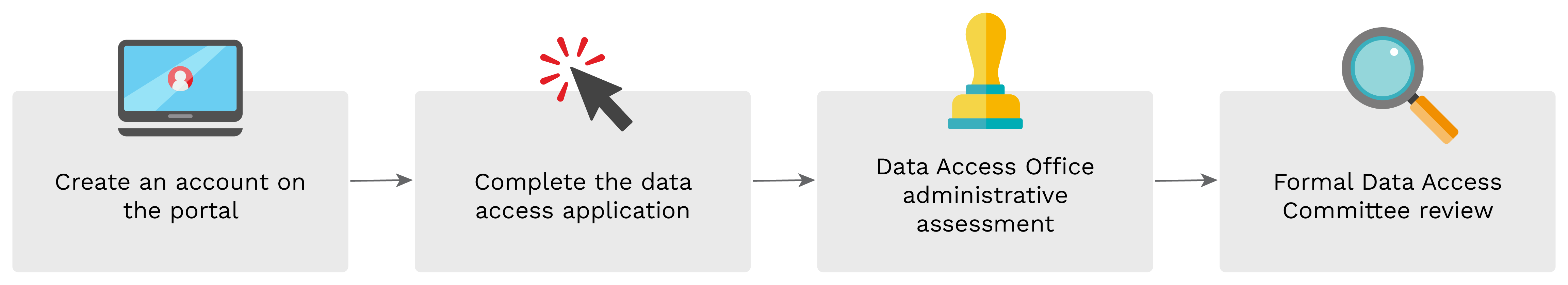
The data access process describes all the steps a researcher must go through to get access to the data, from submission of a research project to final approval and completion.
Data Access Process
Create an account
Before initiating a request for data access, all researchers must [create a user account](/signup).
Complete and submit your access request form
A new data access request is to be created from the [Data Access](/data-accesses) page. Researchers are encouraged to review the **Data Access Policy** and contact the Access Office with any questions they have beforehand [(data@covidimmunitytaskforce.ca)](mailto:data@covidimmunitytaskforce.ca).
When researchers are ready to complete and submit an access request, they must login to their CITF Portal account, create a new access request, fill out and submit the online application form, including all required documentation.
Track your request
Following the submission of the application form, the Data Access Office and Data Access Committee will review and communicate with the researcher if any additional information is needed.
Researchers will be able to track the progress and history of their access requests online, by going to the [Data Access](/data-accesses) page and selecting the data access request. The data access request dashboard summarizes the status of the request and any actions that are required from the researcher. A notification will be sent on approval or rejection of the submitted project.
Project progress and amendments
If there are any changes to the research project or research project personnel from the original Data Access Application that could affect the use of the CITF Data, an Amendment form must be completed before said changes can be carried out by the research team.
Once approved, the title of the Research Project, name(s) of the Approved User(s), their status and credentials, name(s) of the Approved Institution(s), and a lay summary of the scientific abstract submitted by the Applicant will be added to the [Approved Projects](/projects) page on the CITF Website.
For a more detailed view of the Data Access process, please see our detailed **Data Access Procedure**.
Criteria for Approval
Required Documentation
The following documentation is required for administrative approval by the Data Access Office: - Research summary including justification for the requested variables and statistical analyses to be conducted - Evidence of approval by a Research Ethics board for the research project or confirmation for why REB approval is not required - CV of the Researcher applying
Access Criteria and Requirements
Applications are reviewed by the Data Access Committee for compliance with the following: - Data Access Office has received all necessary documents. - Data Access Office confirms availability of CITF Data - Research is in accordance with the guiding principles of the CITF - Research has been deemed scientifically sound and the project scope is appropriate - Justification for the need of the CITF Data is provided - Proof of REB approval, or justification for REB exemption, has been given - Should CDEs (Core Data Elements) deemed “sensitive” be requested, specific justification and study team expertise for the use of these variables is provided in the REB Approval and the Research Summary. Additional information can be found in the CITF Access Policy.
Access Committee
The role of the CITF Data Access Committee (DAC) is to receive and review access requests for projects proposing to use controlled-access CITF data. This is done as per the procedures and criteria outlined in the Data Access Policy. The DAC assesses the feasibility of the proposal and its compliance with the CITF Guiding Principles. - The legal and ethical aspects of health research, data sharing, privacy, and data protection. - The scientific aspects of immunology, population health, or another related scientific discipline. - The technical aspects of database management and/or database security. - Indigenous health research.